Ion exchange chromatography is the separation technique for charged molecules based on their interaction with the oppositely charged stationary phase (ion-exchange resin).
Principe of Ion Exchange Chromatography
- Attraction of oppositely charged resin and analyte.
- Exchange of negatively and positively charged ions to remove charged molecules.
- Stationary phase coated with particular charges binds to mixture components with opposite charges.
- Cation or anion exchange resin with higher affinity binds to charged components, displacing the oppositely charged resin.
- Cation or anion exchange resin-component complex removed using different buffers.
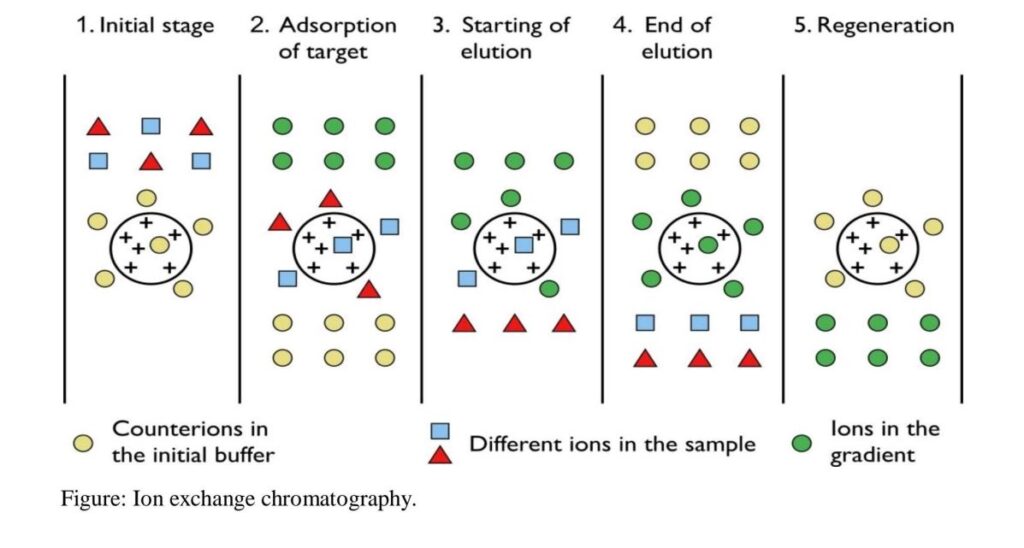
Steps of Ion Exchange Chromatography
- A column packed with charged resin (either positively or negatively charged) is used as the stationary phase.
- The mixture containing charged particles is passed through the column.
- The charged molecules in the mixture bind to the resins with the opposite charge.
- If a cation exchange resin is used, positively charged molecules bind to it, displacing negatively charged ions.
- If an anion exchange resin is used, negatively charged molecules bind to it, displacing positively charged ions.
- An appropriate buffer is applied to the column to separate the complexes of charged exchange resins and charged molecules.
Uses of Ion exchange chromatography
- Purification of water: Positively charged ions are replaced by hydrogen ions, and negatively charged ions are replaced by hydroxyl ions.
- Analysis of products formed after hydrolysis of nucleic acids.
- Separation of metals and other inorganic compounds.
Examples of Ion exchange chromatography
- Separation of positively charged Lanthanoid ions from the earth’s crust.
- Separation of proteins from blood serum.
Ion Exchange Chromatography Types
There are mainly 2 types of ion exchange chromatography.
- Anion exchange chromatography
- Cation exchange chromatography
Anion exchange chromatography
Separation technique for negatively charged molecules using a positively charged stationary phase (ion-exchange resin).
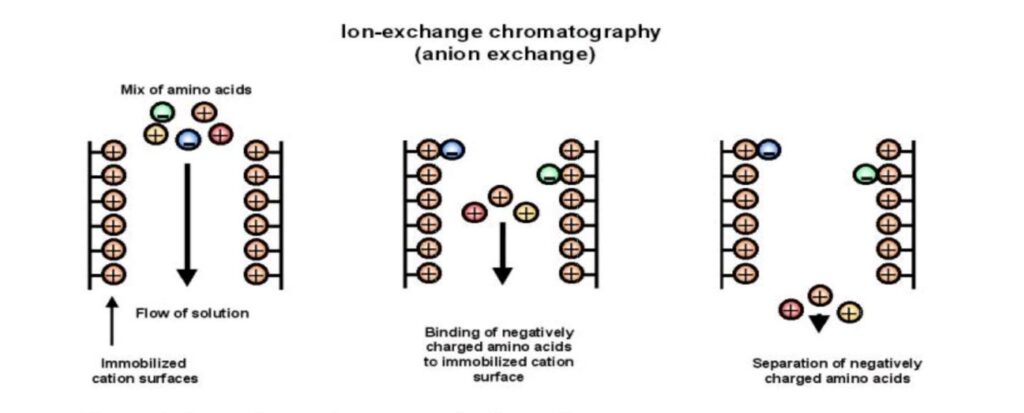
Principle of Anion exchange chromatography
- Based on the attraction between positively charged resin and negatively charged analyte.
- Positively charged ions are exchanged to remove negatively charged molecules.
- The stationary phase is coated with positive charges, and negatively charged components in the mixture bind to it.
- An anion exchange resin with a higher affinity for the negatively charged components then binds to those components, displacing positively charged ions.
- Different buffers are used to remove the anion exchange resin-component complexes.
Steps of Anion exchange chromatography
- A column packed with positively charged resin is taken as the stationary phase.
- The mixture with the charged particles is then passed down the column where the negatively charged molecules bind to the positively charged resins.
- The anion exchange resin is then passed through the column where the negatively charged molecules now bind to the anion exchange resin displacing the positively charged resin.
- Now an appropriate buffer is applied to the column to separate the complex of anion exchange resins and the charged molecules.
Uses of Anion exchange chromatography
- Anion exchange chromatography is used to separate proteins and amino acids from their mixtures.
- Negatively charged nucleic acids can be separated, which helps in further analysis of the nucleic acids.
- This method can also be used for water purification where the anions are exchanged for hydroxyl ions.
- Anion exchange resins can be used for the separation of metals as they usually have negatively charged complexes that are bound to the anion exchangers.
Examples of Anion exchange chromatography
- The separation of nucleic acids from a mixture obtained after cell destruction.
- The separation of proteins from the crude mixture obtained from the blood serum.
Cation exchange chromatography
Cation exchange chromatography is the separation technique for positively charged molecules by their interaction with negatively charged stationary phase in the form of ion-exchange resin.
Principle of Cation exchange chromatography
This technique is based on the principle of attraction of negatively charged resin and the positively charged analyte. Here the exchange of negatively charged ions takes place to remove the positively charged molecules.
Steps of Cation Exchange Chromatography
- A column packed with negatively charged resin is taken as the stationary phase.
- The mixture with the charged molecules is then passed down the column where the positively charged molecules bind to the negatively charged resin.
- A cation exchange resin with a higher affinity to the positively charged components then binds the components, displacing the negatively charged resin.
- The cation exchange resin-component complex is then removed by using different buffers.
Uses of Cation Exchange Chromatography
- Analysis of the products obtained after the hydrolysis of nucleic acids
- Separation of metals
- Purification of water
- Analysis of rocks and other inorganic molecules
Examples of Cation Exchange Chromatography
- Separation of positively charged lanthanoid ions obtained from the earth’s crust
- Determination of total dissolved salts in natural waters by analyzing the presence of calcium ions.
